The first
large-scale CO capture plant will probably be a post combustion process based
on amine absorption, or an oxyfuel process.
But as we
look ahead to 2030 and onwards, new and improved concepts for CO2 capture will
become available. Technologies listed below are currently in various stages of
development and expected to become commercially available in the coming
decades.
Looking ahead to 2030
The
introduction of new technologies always starts with promising prototypes that
are heavily improved as time goes by. This evolutionary improvement is also
expected to be the case with CO2 capture technology.
Consider
the mobile phone. The first prototypes twenty years ago were large, bulky and
expensive. Few people could imagine that this was the beginning of a
development that would alter how people across the globe communicate and
conduct commerce. Today there are more than 5 billion mobile phones, many
affordable to people in the most disadvantaged nations.
A similar
development of CO2 capture technology can also be expected. The first
large-scale CO2 capture plants will probably be based on post-combustion amine
absorption. Soon after oxyfuel and pre-combustion plants will follow.
All these
first-of-its-kind CO2 capture plants will be more capital intensive and less
efficient then subsequent generations. As greater numbers of CO2 capture plants
are installed cost will reduce due to greater operational knowledge and a
refinement of the technologies.
But if we
look ahead to 2030 and onwards there are likely be new alternatives that become
cost-effective offering greater efficiency gains. Some of these future
alternatives include membranes, chemical looping and adsorption (not
absorption!).
Membrane
Membranes
can be used for separating CO2 from other gas components. The technology is
available today but will take some years and further research before it may be
available for large-scale CO2 capture at generation plant.
The
concept is simple. Similar to a filter, components which diffuse more readily
through a material under pressure may be separated out. Generally CO2 capture
membranes are designed to be selective for smaller gasses, for example allowing
Nitrogen to pass through while leaving a pure stream of CO2 behind. Existing
membrane technology currently available does not provide the CO2 purity
necessary for transport. It is possible to increase purity with multi stage
membranes but this also substantially increases the energy penalty required for
repeated compression.
A coal
power plant emits large volumes of CO2 and will consequently require large
areas of membrane. The membrane unit must be small and compact to keep the cost
down. In the figure above this is solved by manufacturing the membrane as thin
hollow fibres. Thousands of such membrane fibres are then combined into a
membrane module in order to obtain a high membrane area within a small volume.
In an
ideal membrane, a high selectivity for the chosen gas will be achieved at low
pressures and low energy costs. With regard to CO2 capture, current research
focus on overcoming challenges related to the material design and improving
membrane performance. Furthermore, energy is required to force gases through
the membrane, and the thicker membrane, the more energy is needed. It is a challenge
to produce membranes as thin as possible to reduce the energy requirements.
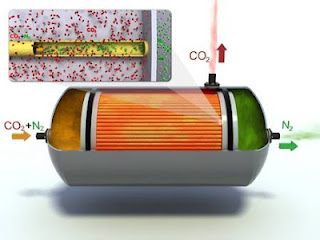
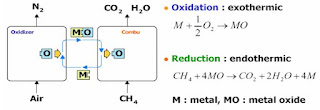
Chemical looping
Chemical
looping is a new technology for combustion with inherent CO2 capture. It is a
significant departure from traditional combustion methods. It is flameless
combustion technology combining two reactors, one air reactor and one fuel
reactor.
In the
air reactor oxygen from the air reacts with a metal-based material to form a
metal-oxide and heat. This metal-oxide becomes an oxygen carrier and is
transferred to the fuel reactor where it reacts with a fossil fuel. The
reaction consumes some heat while producing CO2, water and regenerating the
metal to a pure state. The metal is then recycled to the air reactor and the
cycle repeated.
The
beauty of this process is that it has many of the advantages as oxyfuel, but it
solves the challenges related to oxyfuel. Oxyfuel has the advantage of having a simple and
cheap CO2 separation process, but a challenge is to produce cheap and pure
oxygen. Similar to oxyfuel chemical looping produces a relatively pure stream
of CO2 and water vapour which can be readily separated by a traditional
condensing process.
A major challenge
is the development of robust and efficient oxygen carrier particles. The
reactants must be highly reactive, thermodynamically stable while resistant to
deactivation and degradation. Metal-based materials for reacting with oxygen
and the fuel are currently under development. At present the development of
chemical looping technology is in the early stages and significant development
is needed to commercialise the technology.
Integrated fuel cells
Integrated
fuel cells enable production of the clean energy carriers electricity and
hydrogen from fossil fuel or bio-fuel with ultra-high efficiency and integrated
CO2 capture.
As an
example a process developed by ZEG
Power is described in the following sub-section.
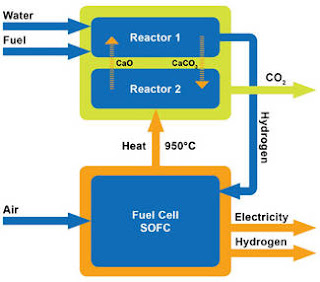
The
electricity is produced from a Solid Oxide Fuel Cell (SOFC) module. The
SOFC module converts fuel to electricity electrochemically without any
conventional combustion. The efficiency of the SOFC module is in the 50% to 70%
range, depending on the operating conditions (ZEG
Power).
Combining
a SOFC with a hydrogen reformer for co-production of electricity and hydrogen
makes a system with a theoretical efficiency of 100%. CO2 capture is enabled
without theoretical energy losses, but some energy losses due to the operation
is however inevitable in real processes. CO2 capture processes require energy,
usually in the form of heat. In an amine process as well as in the ZEG process,
heat is required to release CO2. The same amount of heat is released while the
CO2 is absorbed, but at a lower temperature. When this heat is taken from a
heat powered system, the CO2 capture will reduce the overall efficiency of the
system. In the ZEG process the heat is released at a temperature that is high
enough for the hydrogen reformer, and the waste heat from the CO2 capture
process can be fully utilised in the reformer.
This
particular process also eliminates the need for an afterburner, usually
required in SOFC systems. It further improves the operating conditions for the
SOFC, resulting in higher efficiency and/or lower costs for the SOFC module.
The process completely eliminates the need for combustion, and NOx emissions
are virtually zero. The challenge is to prove the design for larger
(>200Mwh) plants.
Adsorption
As
previously mentioned, the first large-scale CO2 capture plants will probably be
based on post-combustion absorption. But a future alternative to absorption is
adsorption.
During
CO2 capture by absorption a liquid chemical, called solvent, will react with
the CO2, while in adsorption the CO2 will be attached to the surface of a solid
component, called sorbent.
Pressure
swing adsorbtion is a process that utilises the different solubility’s of
gaseous components in a solid. At high pressure, CO2 is adsorbed onto a porous
materials, when the pressure is decreased the gas is desorbed from the porous
sorbent producing a pure CO2 stream. The sorbent can be reused for subsequent
adsorption as the chambers continue to oscillate between high and low pressure
selectively to adsorb and desorb CO2.
The
challenges for adsorption are similar to those related to absorption; finding
new sorbents that react more efficiently and faster with CO2 and that require
less energy to be regenerated. The development of regenerable sorbents that
have high selectivity for CO2 is critical for the adoption of the pressure
swing adsorption process.
CO2
capture by adsorption is not yet a mature commercial technology, but is
currently being tested at a laboratory scale. Research programs are underway
with advances in the technological expected, paving the way for adsorption as a
future solution for CO2 capture.
Source:
BELLONAENVIRONMENTAL


0 comments:
Post a Comment